Osteoclast precursor culture and differentiation
Materials
Dissection
- Clean scissors
- Clean forceps
- 70% Ethanol
- Dissecting board (cork, Styrofoam, etc.)
- Push pins
- Absorptive disposable cloth (like bench liner)
- 10mL syringe (Fisher Scientific – 14-823-2A)
- 25-guage needle (Fisher Scientific – 14-821-13D)
Culture
- α-MEM
- Stericup 0.22-micron PVDF filter with 1L bottle (Millipore – SCGVU11RE)
- 1 jar Minimum Essential Medium powder (Sigma-Aldrich – M0894-10X1L)
- 880mL deionized water
- 10mL 200mM L-glutamine (ThermoFisher – 25030081).
- 10mL 10,000IU Penicillin/10,000μg Streptomycin (ThermoFisher – 15140122)
- 1.98g Sodium Bicarbonate (Fisher Scientific – BP328-500)
- Add 100mL heat inactivated fetal bovine serum
- Mix and sterile filter into a new bottle
- Freezing medium
- 90% α-MEM
- 10% Dimethyl sulfoxide (Fisher Scientific – BP231-100)
- Phosphate Buffered Saline without Ca++ and Mg++ (ThermoFisher – 14190235)
- 100mm tissue culture-treated dishes (Denville Scientific – T1110)
- 60mm untreated suspension culture dishes (Fisher Scientific – 08-772-31)
- 24-well tissue culture plates (Denville Scientific – T1024)
- 50mL and 15mL tubes
- 5mL and 10mL serological pipettes
- 37oC water bath
- ACK lysing buffer (ThermoFisher – A1049201)
- Recombinant mouse Monocyte/Macrophage Colony-Stimulating Factor (MCSF) (Biolegend – 576406)
- Recombinant mouse Receptor Activator of Nuclear Factor κB Ligand (RANKL) (Biolegend – 769404)
- Accutase cell dissociation reagent (ThermoFisher – A1110501)
- Trypan blue
Method
Day 1
- Sacrifice a mouse (C57Bl/6 work well) via CO2 inhalation followed by cervical dislocation
- Using clean technique, dissect out and separate the tibiae and femora.
- Cut the ends off femora and tibiae to facilitate flushing
- Working in the hood, fill a 10mL syringe with 10mL α-MEM.
- Pick up a bone with forceps and insert syringe needle into one end.
- Flush ~2.5mL α-MEM through the bone into a 15mL tube. Note: you’ll know you’ve flushed out the marrow if the bone changes from a reddish color to a more white color.
- Repeat the flushing procedure for remaining bones adding all marrow flushes from each mouse into a single tube.
- Using a 10mL serological pipette, transfer medium with marrow cells into a clean 15mL tube. Take care not to transfer any debris from the bone that may have settled to the bottom of the tube.
- Pellet cells at 300RCF for 5 minutes.
- Aspirate medium and resuspend pellet in 500μL ACK lysing buffer and incubate at 37oC for 2 minutes to lyse red blood cells.
- Add 10mL PBS to cells and pellet at 300RCF for 5 minutes.
- Resuspend pellet in 10mL α-MEM and transfer to a 100mm tissue culture-treated dish.
- Incubate plate in a 37oC humidified incubator at 5% CO2 This will allow osteoblasts, stroma, fibroblasts, and all other adherent cells that may be in the flush to attach to the plate.
Day 2
- Transfer non-adherent cells to a 15mL tube.
- Add α-MEM to a volume of 10mL.
- Add MCSF to a final concentration of 25ng/mL.
- Deposit cells to 100mm petri dish (non-culture treated, non-pyrogenic).
- Incubate dishes in a 37oC humidified incubator at 5% CO2 overnight.
Day 3
- Refresh medium on cells.
Day 5
- When plates are 75% confluent, wash with PBS, and add 1mL Accutase. It usually takes ~5min at room temperature for Accutase to weaken the attachment enough to lift the cells.
- Add 4mL α-MEM to cells and use gentle pipette motion to lift. Move cells to a 15mL tube.
- Make a 2-fold dilution of cells (15μL cells + 15μL trypan blue) and count cells using a hemocytometer.
- To make osteoclasts in the following plates use these numbers of macrophages (~26000 cells per cm2 of culture area):
- 96-well culture treated plate – 10 x 103 (seed in 200μL)
- 24-well culture treated plate – 5 x 104 (seed in 1mL)
- 35mm culture treated dish – 2.5 x 105 (seed in 2mL)
- 60mm culture treated dish – 7.4 x 105 (seed in 3mL)
- 100mm culture treated dish – 2 x 106 (seed in 10mL)
- Prepare a sufficient volume of cells for seeding and add MCSF to a final concentration of 25ng/mL and RANKL to a concentration between 6.25 and 25ng/mL.
- In our experience, 25ng/mL RANKL produces maximal osteoclast differentiation. If you expect your experimental group to demonstrate enhanced differentiation, use a lower concentration.
- Alternatively, cells can be seeded on bone or dentine slices. Use the same cell density of the culture vessel the slice is kept in (usually a 24-well plate)
- Incubate dishes in a 37oC humidified incubator at 5% CO2 for 2 days.
Day 6
- Refresh the medium on the cells – α-MEM w/ 25ng/mL M-CSF + 6.25-25ng/mL RANKL
- Late in the afternoon, you may see some multinuclear cells.
Day 7
- Osteoclastogenesis should be complete in the AM
- Osteoclasts don’t live long in culture, so watch your cells closely.
- Osteoclasts on bone slices usually take 1 more day to mature than those on culture plastic.
- Osteoclasts can be identified using a TRAP stain (sample staining protocol).
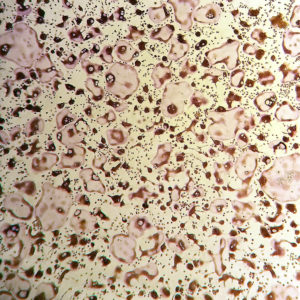
Mature osteoclasts stained for Tartrate-Resistant Acid Phosphatase (TRAP) activity.

